ASTR 1210 (O'CONNELL) SUPPLEMENTS
There are three special supplements which are required reading for this class. Supplement I (a PDF file) contains reference material on units of measurement, powers of ten, nomenclature, and special symbols. It should be consulted as necessary throughout the remainder of the course. Supplements II and III are intended to replace the material in the textbook covering the basic properties of matter, the nature of forces, the electromagnetic spectrum, and how astronomers exploit the properties of the EM spectrum to deduce the physical nature of distant objects. They also serve as the Study Guides for the lectures where this material is covered. Most of the concepts presented in II and III should already be familiar to you. These supplements are available in this HTML file.
ASTRONOMY 1210: SUPPLEMENT II
A. Elementary Particles
You should already be familiar with three elementary particles: the electron, the proton, and the neutron. These are the primary constituents of atoms. Atoms were first proposed as the basic components of matter by Greek philosophers (e.g. Democritus, ca. 400 BC), and their reality was debated for thousands of years. Their existence was proven beyond doubt in the 19th century. Protons and neutrons are bound together in the nucleus of the atom, an object about 10-13 centimeters in diameter. The electrons orbit the nucleus in a cloud which is 100,000 times larger, 10-8 cm in diameter. There is a multitude of other elementary particles, most of which exist only for very short periods of time; most of these will not be discussed further in the course.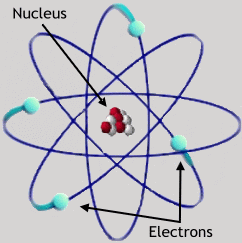
- Mass of proton (or neutron) = 1.7 x 10-24 grams
- Mass of electron = mass of proton/1800
-
All elementary particles which have been isolated are found to have only
certain definite amounts of charge: none, exactly one positive unit
of charge, or exactly one negative unit. (Protons and neutrons
are constructed of even more basic subunits
called "quarks", which have fractional charges. But these are strongly
bound together inside protons and neutrons. Free quarks
should rarely exist and have never been observed in the laboratory.)
- The neutron has zero charge
- The proton has a charge of +1
- The electron has a charge of -1 (Note: The + or - sign is simply a convention indicating that electrons and protons behave differently in EM force fields.)
-
Note: the abundances of elements on the Earth are very different from
their cosmic abundances (i.e. those in the stars or the
interstellar gas). 75% of the earth's crust is oxygen or
silicon. However, in our galaxy, hydrogen makes up about 72% of the
total mass, helium another 25%, and all other elements only 3%.
B. Forces
If particles did not exert forces on each other, there would be no organization in the universe: no planets, no stars, no people. Particles would move throughout space in random but unchanging paths, never altering their motion even when they passed through one another. However, particles do exert forces on each other, and the nature of these forces depends on the mass, charge (and other "internal" properties we will not discuss), and state of motion of the particle. Isaac Newton was the first to formulate a quantitative description of how matter responds to forces which are applied to it. Historically, Newton's laws were a major stimulus for both the scientific revolution and the industrial revolution since they demonstrated that the universe is understandable through rational analysis. His description is encapsulated in his second law of motion :-
F = m a
-
a = F / m .
-
This expression implies that for a given mass, the larger the force
the larger the acceleration. Also, for a given force, the larger the
mass the smaller the acceleration. A boxcar is more massive than your
little brother and is therefore harder to push around.
As the rate of
change of a quantity (velocity), acceleration is the mathematical
derivative of velocity (which is itself the derivative of
position). Also, the force may depend on position, velocity, and
other properties of the system. Thus, this expression is actually
not a simple algebraic expression but instead is a differential
equation. In order to solve it, Newton was forced to develop
calculus, one of the most powerful and valuable intellectual
tools ever invented.
Gravity
The gravitational force is an attractive force between any two objects containing mass. All particles in the universe attract each other; any pair of particles which start from rest will eventually move together (in the absence of other nearby particles). Gravity seems strong and inexorable in everyday life, but in fact it is by far the weakest of the known forces. Gravity falls off with distance, but is a long-range force compared to the nuclear forces (e.g. it can act over intergalactic distances).-
Newton was the first to develop a quantitative theory of gravity. In
Newton's formulation, gravitational force acts along the line
connecting the two objects and is proportional to the
product of the masses of the two objects but inversely proportional to
the distance between the two squared. That is,
-
Fgrav = G M1 x M2 / R2
Nuclear Force
This is a short range force, acting over distances of only 10-13 cm (i.e. about the size of an atomic nucleus). It has no direct influence over significantly larger distances. (The longer range effects of nuclear materials, e.g. in radiation therapy, are produced by particles or photons ejected from atomic nuclei by the nuclear forces, not the forces themselves.) Because this is the case, the effects of nuclear force are described by the quantum theory and not Newton's Second Law. Technically, nuclear force is subdivided into the strong and weak forces (and hence you will often find references to the "four forces" of nature, rather than the three described here).-
Inside the nucleus, nuclear forces are very strong, some
1040 times stronger than gravity(!), which is wholly
negligible in nuclei. The nuclear forces bind protons and neutrons
together in nuclei. They are responsible for nuclear power,
radioactivity, and the explosive energy of nuclear bombs.
[Note that "atomic bomb" is really a misnomer, since it is not atoms
that release the energy in a fission or fusion bomb but only their
nuclei. Chemical explosives (e.g. dynamite) are the real
"atomic bombs" because their energy release originates from changes in
the electron shells around the nuclei.]
Electromagnetic Force
The ubiquity of the effects of electromagnetic (EM) force make it the most important in everyday life. It is, of course, responsible for electricity and magnetism. But it is also the source of combustion, solidity, friction, capillary action, etc. In short, it holds our everyday world together. EM force is responsible for all chemical reactions, including those occurring in biological organisms.-
The simplest form of EM force is the electrostatic force ,
which acts between any two objects with net charge which are at
rest. If the two charges have the same sign (both + or both -), the
force is repulsive and the objects tend to move apart. If the two
charges are opposite, the force is attractive (like gravity). The ES
force is a strong force: the ES repulsion between two electrons
is 1036 times stronger than the corresponding gravitational
attraction. It is also in principle a long range force--but
not in practice, since macroscopic objects tend to have zero net
charge.
The magnetic force is more complicated. It acts in a complex
manner on moving charged particles. Two objects with zero net
charge can interact magnetically if charges inside of them move in
sufficiently organized patterns ("currents"). A bar magnet is an
example of an object with zero net charge which can be affected by
external magnetic fields. The Sun, the Earth, and most other planets
also have fairly strong magnetic fields, which affect the motion of
particles near them. On the Earth's surface the most familiar
magnetic effect is found in the orientation of a compass needle to the
Earth's "magnetic poles". Magnetic forces are also long
range but are much more important on galactic scales than are
electrostatic forces because they are usually not self-shielding like
ES forces. On such scales they are usually less important than
gravity, however.
Notes:
-
We have never been able to identify any special interaction (e.g. a
"life force") that is harbored only by living beings and governs their
functioning, behavior, or mental processes. EM forces control all biological
phenomena, including
consciousness.
EM force is the only kind of interaction which could be fashioned into
the mysterious "force fields" favored by science fiction writers or
could be responsible for claimed borderline phenomena like
telekinesis, extra-sensory channels of communcation, etc.
-
When two atoms approach each other, their constituents feel a
combination of attractive and repulsive forces, depending on the exact
configuration of the nuclei and electrons. Under the right
circumstances, atoms can bond together---i.e. the attractive
force overcomes the repulsive force---and create a stable system.
This is called a molecule. Most molecules are relatively
simple, consisting of only a few atoms (water consists of only three),
but organic molecules can be of enormous size, containing thousands to
millions of individual atoms.
Chemical energy is released when the electronic configuration
of molecules is altered through interactions between them.
Fire is perhaps the most obvious everyday example of chemical
energy release.
ASTRONOMY 1210: SUPPLEMENT III
A. Electromagnetic Waves
Based on the experimental studies of Faraday and others, especially Faraday's discovery of electromagnetic induction and his concept of a magnetic force field, James Clerk Maxwell managed to summarize (ca. 1865) all electromagnetic phenomena in a set of elegant and interconnected equations now known as Maxwell's equations. These represent the third great generalized physical law, the first two being Newton's equations of motion and his theory of gravity. Maxwell was able to show that his equations implied that electric and magnetic force fields can propagate through space. A moving electron, for example, sends out a disturbance which can cause other electrons to move; "target" electrons at successively greater distances will feel the disturbance at successively later times. The propagating disturbance is similar to a water wave produced by a rock dropped into a pond and is therefore called an electromagnetic wave. One of Maxwell's great discoveries was that he could use his equations to predict the velocity of an EM wave as it propagated through a vacuum. He found the velocity to be the same as the speed of light, which had already been determined with fair precision. The immediate inference was that light is one kind of electromagnetic wave. Maxwell's theoretical prediction of EM waves was later confirmed experimentally by Heinrich Hertz (1887), who detected, for the first time, artificially generated radio waves. Only a few years later in 1895, the physicist Wilhelm Röntgen discovered high energy EM waves in the form of X-rays and simultaneously recognized their tremendous penetrating power and usefulness as medical diagnostics. Maxwell's prediction of EM waves has had profound practical consequences.
B. The Electromagnetic Spectrum
All frequencies from zero to infinity are possible for EM waves, and this total range is called the electromagnetic spectrum. See the figure below; the textbook presents other illustrations and more information. Radio, television, microwaves, infrared, visible light, ultraviolet light, X-rays, and gamma rays are all forms of EM waves. All of these waves travel at the same speed (at least in a vacuum): the speed of light, which is 300,000 kilometers per second (186,000 miles per second).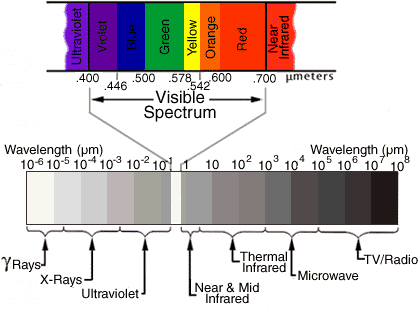
(Units marked are microns. 1 micron = 10,000 Å)
 Applet. Here
is an interactive Java applet illustrating wave diffraction.
Applet. Here
is an interactive Java applet illustrating wave diffraction.
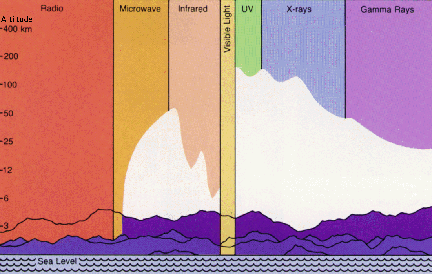
for different kinds of EM waves
-
Sound is not an EM wave. A sound wave is a
compressional disturbance in a medium (air, water, etc.) and
propagates far more slowly than EM waves. Sound waves cannot
exist in a vacuum, whereas EM waves can.
- The difference in propagation speed between sound and light is
vividly apparent during a thunderstorm. The light of a lightning
flash will reach you almost instantaneously, whereas the sound travels
at a speed of only 1100 feet per second, so there will be an
appreciable lapse between the flash and the sound of the corresponding
thunder. To estimate the distance of the lightning flash (in feet),
simply multiply the number of seconds between the flash and the sound
arrival time by 1100. A 5-second delay means the lightning strike
was over a mile away.
C. Photons
Although EM radiation does have many wavelike properties, it is not simply a wavelike disturbance. It is absorbed or emitted from particles in tiny packets, called photons , which themselves behave more like particles than waves. The energy of a photon depends on its frequency (in the wave analogy): the higher the frequency, the larger the photon's energy. Photons are created or destroyed by atoms through interactions with the charged particles within the atom. According to the quantum theory, the internal energy states of an atom are discrete or quantized. This means that the electron cloud around the nucleus of the atom can exist only in certain definite energy states. Whenever the cloud changes its energy state, a certain definite amount of energy must be lost (if the energy drops) or gained (if the energy increases). One of the most important ways in which atoms lose or gain energy is through photons. The electron cloud can spontaneously lose energy by emitting a photon, which then escapes from the atom. Alternatively, it can absorb a photon which is near the atom, thereby gaining energy (and destroying the photon).D. Spectroscopy
As a consequence of sponteneous photon creation by atoms, all objects which are above a temperature of absolute zero emit electromagnetic waves---even textbooks and ice cubes. There is a great deal of information about the physical properties of the object contained in the distribution of wave energy as a function of wavelength or frequency in its electromagnetic spectrum. For short, this distribution is called the object's spectrum. "Spectroscopy" is the study of EM spectra. The spectrum of a dense object like the Earth or Lake Superior or the inner regions of the Sun is smooth or "continuous" and changes only slowly with wavelength. You can think of the atoms and electrons in such an object as interacting so strongly that the individual signatures of each type of atom are blended away. Such an object is said to be in thermal equilibrium, and the resulting spectrum is sensitive only to its temperature. This kind of spectrum is called a blackbody spectrum, named after ideal, laboratory sources which are purely self-luminous and reflect no light. Such sources have a spectrum with a smooth, well-defined, characteristic shape, as shown in the figure below: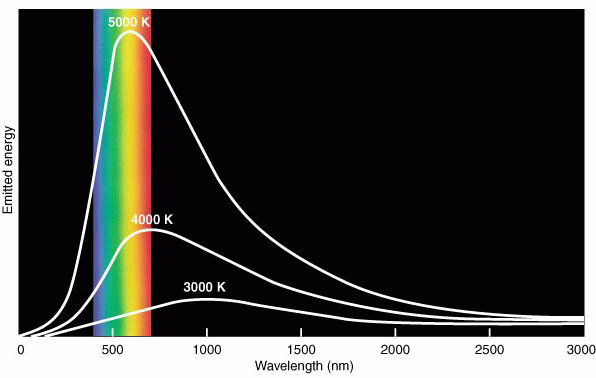
(The wavelength scale is in nanometers. 500 nm on this scale corresponds to 5000 Å or 0.5 microns.) The colored bands show the "visible" part of the EM spectrum.
-
Does this fact, combined with the principle of natural biological
selection, suggest a reason why our eyes are most sensitive to
yellow-green light?
An incandescent light bulb operates by using an electric current to
heat a thin metal filament until it glows brightly, at about
2500o Kelvin. Higher temperatures would vaporize the
filament. Hence, incandescent light bulbs are redder than the Sun
and, in fact, redder than any of the stars visible to the unaided eye,
which all have temperatures above 3000o Kelvin.
- Objects at room temperature (about 300o Kelvin) also
emit their peak radiation in the infrared. They
are self-luminous at those wavelengths, but the light we can
detect from them with our eyes is not their own self-radiation
but rather
reflected light from higher temperature sources (such as the
Sun or incandescent light bulbs).
For instance, see the image below, which compares the visible-band
(reflected light) with the "thermal-infrared" appearance of a cat
(with a body temperature of about 312 Kelvin).

The total radiative output from a blackbody source (summed over all wavelengths) is a very strong function of its temperature and scales as the temperature (on the Kelvin scale) to the fourth power (T4).
-
Here is a Java demo of the effects of temperature on EM
radiation.
-
If you could pump up a single atom to a high energy state and then
observe it sitting quietly without further outside disturbance, you
would find that it would emit a series of photons with discrete, but
different, energies as it lost energy from its electron cloud. Thus,
its spectrum would consist of a series of sharp peaks: only certain
frequencies or wavelengths would be included. It would be faint (or
dark) except at the peaks. This is called an "emission line
spectrum" because if you plotted the number of emitted photons versus
their frequencies, it would resemble a picket fence.
If you imagine the same atom left in a low energy state but placed
in front of a dense, glowing object, you would observe a spectrum which
was the reverse of the atom's emission line spectrum. Here, you
would see light from the background glowing object at all frequencies
except where the object's photons could be absorbed by the electron
cloud. This is an "absorption line spectrum": dark at those
discrete frequencies where the
electron cloud can absorb energy but bright elsewhere.
The 3 general kinds of spectra are summarized in the illustration
below:
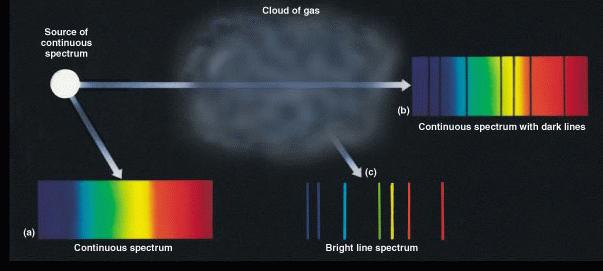
The essential practical implication of these facts stems from the fact that the spectrum of each type of atom is different because its electronic structure is different. A hydrogen atom has a different spectrum than a helium atom, and carbon atoms have a yet different spectrum. Obviously, then, the emission or absorption line spectrum of a star or planet is related to its chemical composition.
-
It is, in fact, through analysis of such spectra that we know the
chemical content of stars, the interstellar gas, and other galaxies
even though they are tremendously far away.
For instance, the figure below shows part of the spectrum of the Sun
at yellow wavelengths. The dark lines are produced by atoms of
magnesium, iron, titanium, and other elements in the cooler, outer
layers of the Sun which are seen against the bright continuum produced
by its hot inner regions. Quantitative analysis of such spectra tells
us the detailed chemical makeup of the Sun. For example, the
element helium (the second lightest after hydrogen) was
discovered in the spectrum of the Sun before it was studied in
an Earth-bound laboratory. Click on the image for an enlarged
spectrum of the Sun.

-
Bad Philosophy Footnote: Click here for
a related description of one of the worst, but not one of the last,
faulty prognostications about science by a philosopher.
E. Luminosity and Flux
The luminosity of an object is defined to be the total amount of electromagnetic energy radiated by the object in a given time. It may refer to the whole EM spectrum, in which case it is denoted a "bolometric" luminosity, or it may refer only to a limited part of the spectrum. For example, the "visual" luminosity of a star refers to the total energy output in the visible part of the spectrum. It is denoted LV. Luminosity is an intrinsic property of the object; that is, it does not depend on how far away the observer is. "Intrinsic brightness" or "power" are synonyms for luminosity. In the cgs system, luminosity is measured in ergs per second. The response produced by an object of given luminosity in a detector (for example the human eye) depends both on the luminosity and the distance of the object. More distant objects produce smaller responses. The response of a detector to a given source of EM radiation is a function of the flux of radiation from the object. The flux is defined to be the energy crossing a given area in a given time. It is measured in ergs per second per square cm (ergs/sec cm2) in the cgs system. Flux is not an intrinsic property of the source because it depends on the observer's distance. The greater the flux, the greater the response of a given instrument. Flux can be defined with respect to part or all of the EM spectrum. A little thought will lead you to realize that flux = (total energy output of source per unit time)/(area of sphere passing through observer with center on source). Or,-
F = L / (4 x pi x R2)
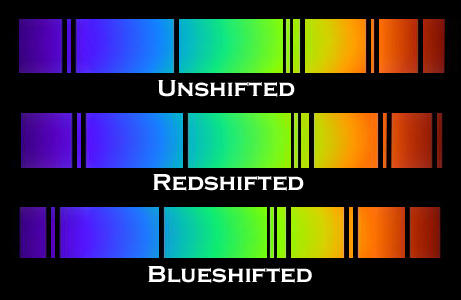
F. The Doppler Effect
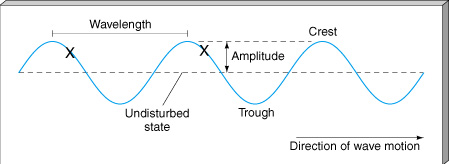
The Doppler Effect is a change in the apparent wavelength or frequency of an EM wave caused by the motion of the source of the wave toward or away from the observer. It also occurs with sound waves, where it is most familiar as the change in pitch of a police or ambulance siren as it passes you on the street. When the siren is approaching you, the pitch is higher (i.e. the frequency is higher and the wavelength is shorter) than when it is receding from you (lower frequency, longer wavelength). The Effect is explained by the fact that if a source is approaching you while emitting a wave of fixed wavelength, each successive crest of the wave starts from a position a bit closer to you than the preceding one. But the crests still travel at the same velocity. This means that the distance between successive crests (the wavelength) traveling in your direction will be smaller than if the source were stationary. The stream of crests moving in your direction is therefore more "compressed" than for a source at rest. You will observe a signal with shorter wavelength (higher frequency) than that from the same source at rest. Conversely, if the source moves away from you, the stream of crests is "stretched", and you will observe a longer wavelength (lower frequency). "Doppler radar" makes use of this phenomenon to measure the motion of air streams in the atmosphere or of automobiles on the highway (often in the hands of the state police). Here
is a nice Java applet illustrating the Doppler Effect. You can
interactively adjust the velocity and direction of motion of the
source. Note how the propagating waves are compressed along
the direction of motion but expanded along the opposite direction.
The magnitude of the effect depends on the velocity of the source.
If the source moves faster than the speed of propagation in
the surrounding medium, a cone-like "shock wave" results; this is what
produces a "sonic boom" from a supersonic jet plane in the atmosphere.
However, since no source can move faster than the speed of light in a
vacuum, this case doesn't apply to light waves emitted by objects
moving in space.
Here
is a nice Java applet illustrating the Doppler Effect. You can
interactively adjust the velocity and direction of motion of the
source. Note how the propagating waves are compressed along
the direction of motion but expanded along the opposite direction.
The magnitude of the effect depends on the velocity of the source.
If the source moves faster than the speed of propagation in
the surrounding medium, a cone-like "shock wave" results; this is what
produces a "sonic boom" from a supersonic jet plane in the atmosphere.
However, since no source can move faster than the speed of light in a
vacuum, this case doesn't apply to light waves emitted by objects
moving in space.
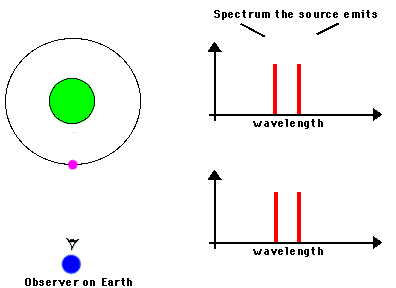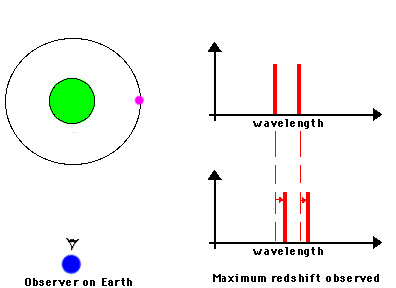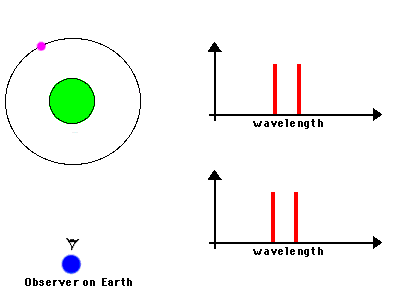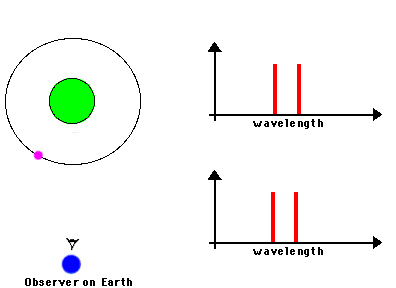
Reading for this lecture:
-
Supplements II and III
Optional reading: Bennett textbook, Ch 5
-
Study Guide 11
Bennett textbook: Chapter 7 except pp. 202-212; Chapter 8; "Summary
of Key Concepts" for Chapter 13.
- Supplement I: Powers of Ten/Special Symbols (PDF)
-
Background info on electromagnetic waves
-
Background info on spectral analysis
- Astronomical Spectroscopy (Kaler, UILL)
 Previous Guide
Previous Guide
|
 Guide Index
Guide Index
|
 Next Guide
Next Guide
|